|
4. "Perfect" fullerenes Using the mentioned connection between alternating knot or
link diagrams and 4-regular (chemical) Schlegel diagrams of fullerenes,
it is interesting to consider all of them after such conversion. For example,
two chemical isomers of C20 will result in knots, and from 7
isomers of C24 we obtain four knots, one 3-component, one 4-component
and one 5-component link. Among the links obtained, two of them (3-component
and 5-component one) contain a minimal possible component: hexagonal carbon
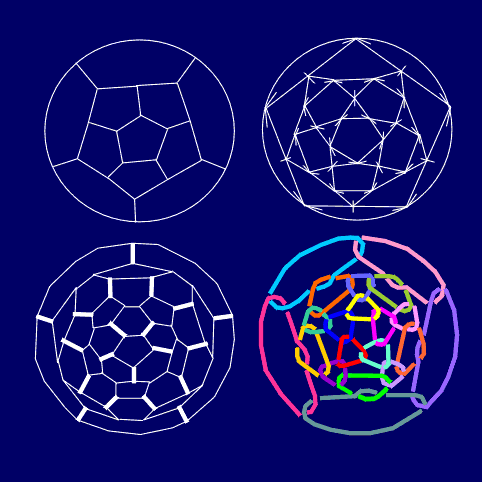
ring (or simply, a circle). It is interesting that C60 consists
only of such regularly arranged carbon rings, so maybe this could be another
additional reason for its stability (Fig.
7). Therefore, it will be interesting to consider the infinite class
of 5/6 fullerenes with that property, that will be called "perfect". Some
of "perfect" fullerenes are modeled with hexastrips by P.Gerdes [13],
and similar structures: buckling patterns of shells and spherical honeycomb
structures are considered by different authors (e.g. T.Tarnai [14]).
 To obtain them, we will start from some 5/6 fullerene
given in geometrical form (i.e. by a 3-regular graph). Than we could use
"mid-edge-truncation" and vertex bifurcation in all vertices of the triangular
faces obtained that way, transforming them into hexagons with alternating
digonal edges. Let is given some fullerene (e.g. C20) in its
geometrical form (i.e. as 3-regular graph). By connecting the midpoints
of all adjacent edges we obtain from it the 3/5 fullerene covered by connected
triangular net and pentagonal faces preserved from C20. After
that, in all the vertices of the truncated polyhedron we introduce digons,
to transform all triangles into hexagonal faces. This way, from C20
we derived C60 (in its chemical form) (Fig.
7). The mid-edge-truncation we could apply to any 5/6 (geometrical)
fullerene, to obtain new "perfect" (chemical) fullerene, formed by carbon
rings. This way, from a 5/6 fullerene with v vertices we always
may derive new "perfect" 5/6 fullerene with 3v vertices (Fig.
8). Moreover, the symmetry of new fullerene is preserved from its generating
fullerene. According to the theorem by Grünbaum & Motzkin [15],
for every non-negative n6 unequal to 1, there exists
3-valent convex polyhedron having n6 hexagonal faces.
Hence, from the infinite class of 3-regular 5/6 polyhedra with v=20+n6
vertices, we obtain the infinite class of "perfect" fullerenes with v=60+3n6
vertices. The "perfect" fullerenes satisfy two important chemical conditions:
(a) the isolated pentagon rule (IPR); (b) hollow pentagon rule (HPR). The
IPR rule means that there are no adjacent pentagons, and HPR means that
all the pentagons are "holes", i.e. that every pentagon could have only
external double bonds. The first 5/6 fullerene satisfying IPR is C60,
and it also satisfies HPR. The IPR is well known as the stability criterion:
all fullerenes of lower order (less than 60) are unstable, because they
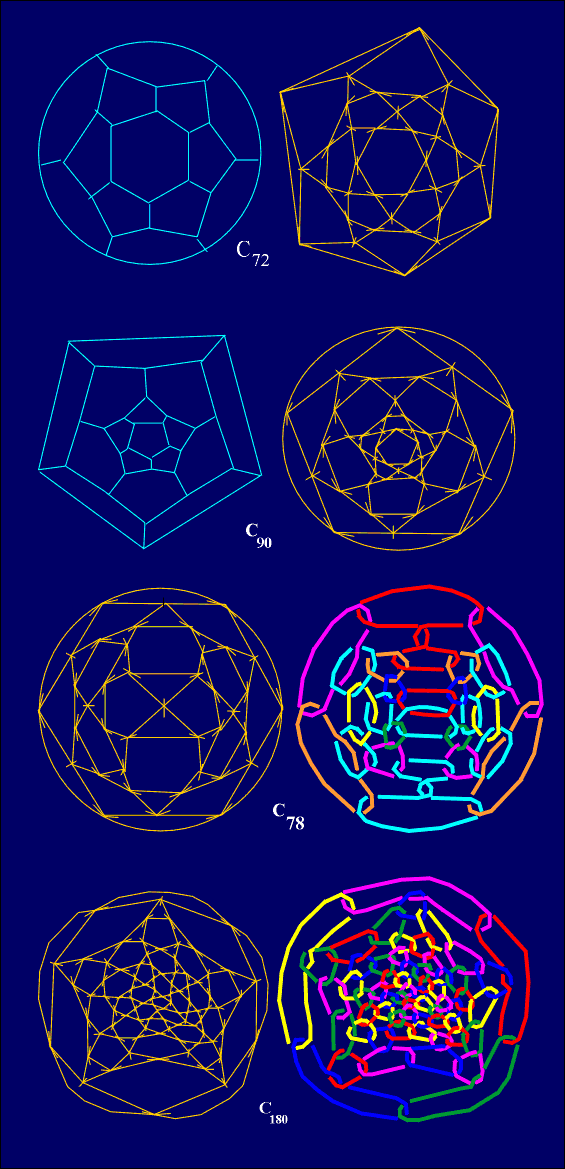
don't satisfy IPR. On the other hand, C70 satisfies IPR, but
cannot satisfy HPR (Fig. 9).
 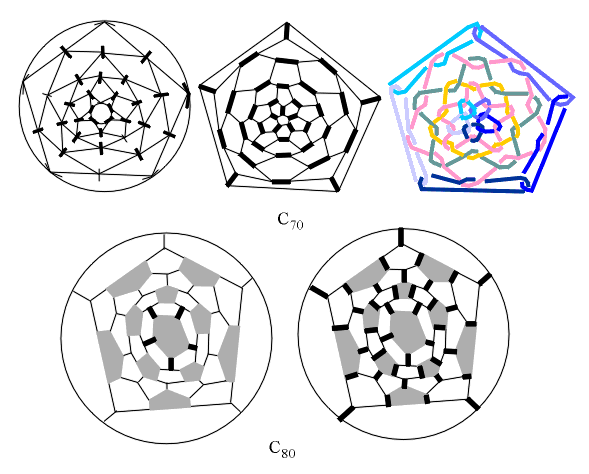 The same situation is with C80, possessing
the same icosahedral geometrical symmetry as C60, but not able
to preserve it after edge-coloring, because HPR cannot be satisfied (Fig.
9). This is the reason that only "perfect" fullerenes, with G=G'=
[3,5]=Ih=S5,
satisfying both IPR and HPR will be C60, C180,
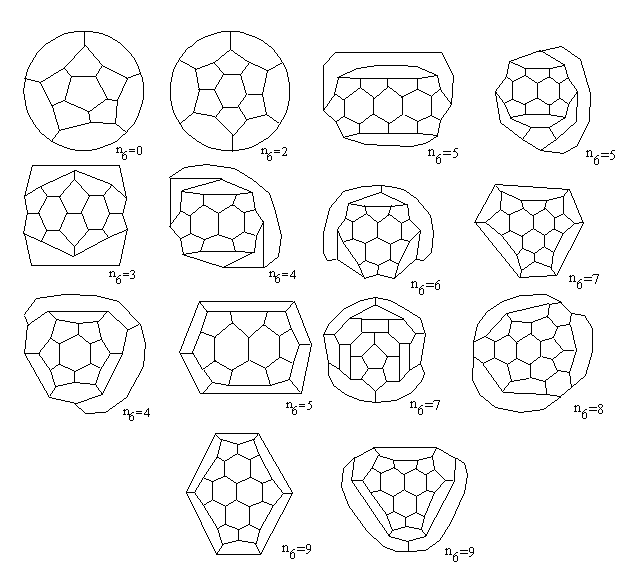
C240, etc. We need also to notice that for n6=
0,2,3 we have always one 3-regular 5/6 polyhedron (i.e. geometrical form
of C20, C24, C26), but for some larger
values (e.g. n6= 4,5,7,9) there are several geometrical
isomers of the generating fullerene, and consequently, the same number
of "perfect" fullerenes derived from them (Fig.
10). Hence, considering the fullerene isomers, we could distinguish
"geometrical isomers", this means, different geometrical forms of some
fullerene treated as 3-regular 5/6 polyhedron, and "chemical isomers" –
different arrangements of double bonds, obtained from the same 3-regular
graph by its edge-coloring.
 |